organics.synthesia.eu / Pharmacy / Mitoxantrone hydrochloride
Mitoxantrone hydrochloride
The production of the active pharmaceutical substance Mitoxantrone hydrochloride at Synthesia has more than 20 years of tradition. Mitoxantrone is further used for the manufacture of dosage form which is used in treatment of patients, especially those who have been diagnosed with cancer. Despite the fast development in the treatment of cancer, Mitoxantrone maintains its position amongst the drugs in the treatment of certain types of cancer.
This drug belongs to the group known as cytotoxic or antineoplastic.
We offer high quality product - USP, PhEur, production under strict GMP conditions, full registration support (DMF), prompt deliveries and customer service.
| Trade Name: | Mitoxantrone hydrochloride | |
| Chemical Name: | 1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione dihydrochloride | |
| CAS: | 70476-82-3 | |
| EC: | 274-619-1 | |
| Synonyms: | 1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]anthraquinone dihydrochloride | |
| Molecular Formula: |
C22H28N4O6 . 2 ClH |
|
| Structural Formula: |
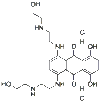
|
|
| Molecular Weight: | 517.40 | |
| Characteristics: |
dark blue, hygroscopic crystalline powder, odourless |
Packaging
Brown glass bottle 25 g
Storage
To be stored at room temperature, in original, tightly closed dark glasses provided with external threaded closures, protected against light, heat, moisture and frost. It has shelf life of two years, provided the specified storage conditions have been observed.
PF 2026
Synthesia at the fair CPhI 2025
Synthesia at the fair Chemspec Europe 2025
